According to the Rate Law How Do Concentrations Affect Rate
According to rate law rate of a reaction depends upon the concentration of the species participating in the reaction. The notation A is read as the molar concentration of Reactant A.
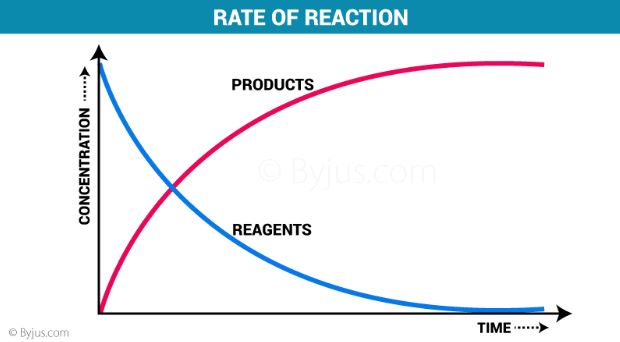
Rate Of Reaction Definition And Factors Affecting Reaction Rate
How do concentrations affect rate according to the rate law.
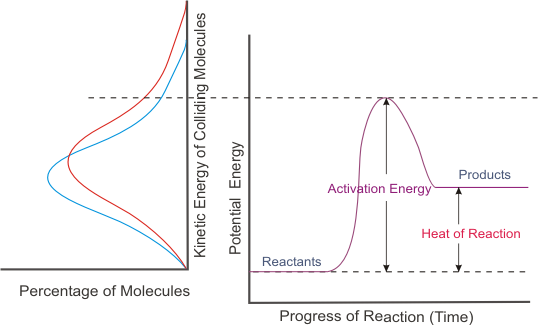
. D the rate increases as concentrations increase. OSMOSIS LAB REPORT INSTRUCTIONS Write the report in the past tense ALL the way through. Due to this there will rapid formation of products.
Typically reaction rates decrease with time because reactant concentrations decrease as reactions are converted to products. The rate increases as concentrations increase. R kAxBy r k A x B y.
What is the rate of a reaction if the value of k is 3 and A and B are each 2 M. The questionHow different concentrations of sucrose affect the rate of. The rate law uses the molar concentrations of reactants to determine the reaction rate.
How do concentrations affect rate according to the rate law. For example Rate Therefore when there is increase in concentration of reactants then there will be more number of collisions. Due to this there will rapid formation of products.
The rate increases as concentrations decrease. Typically increased concentrations of reactants increases the speed of the reaction because there are more molecules colliding and reacting with each other. According to rate law rate of a reaction depends upon the concentration of the species participating in the reaction.
In general a rate law or differential rate law as it is sometimes called takes this form. Elaborate on the effect of solute concentration on the rate of osmosis. Hence rate of reaction will increase.
As described in the previous module the rate of a reaction is affected by the concentrations of reactants. For example Rate. Reaction rates generally increase when reactant concentrations are increased.
For the general reaction aAbB C aA bB C with no intermediate steps in its reaction mechanism meaning that it is an elementary reaction the rate law is given by. How does the rate law show how concentration changes affect the rate of reaction. Therefore when there is increase in concentration of reactants then there will be more number of collisions.
Correct answer to the question According to the rate law how do concentrations affect rate. In this equation A and B express the concentrations of A and B respectively in units of moles per liter. The rate increases as concentrations increase.
The rate is independent of concentrations. Rate laws or rate equations are mathematical expressions that describe the relationship between the rate of a chemical reaction and the concentration of its reactants. The reactant concentrations decrease.
A definition of osmosis in terms of solute concentration would be a good background information. According to the rate law how do concentrations affect rate.

Factors Affecting Reaction Rates

Rate Law And Reaction Order Video Khan Academy
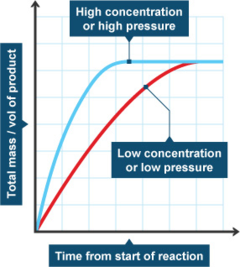
Chemical Reactions Rates Of Reaction Factors Pathwayz

Rates Of Reaction Notes Photos July 2011 Cxc Chemistry Lessons Chemistry Lessons Chemistry Lesson
No comments for "According to the Rate Law How Do Concentrations Affect Rate"
Post a Comment